Transparency in the Fresh Food Industry: Holding Brands Accountable
Demand accountability in the fresh food industry as brands like Darwin’s and Answers face scrutiny for pathogen contamination.
Demand accountability in the fresh food industry as brands like Darwin’s and Answers face scrutiny for pathogen contamination.
Uncover how canine health research is revolutionizing life for both pets and people with exciting discoveries!
If you’re just catching up, Midwestern Pet Foods (manufacturer of Earthborn, ProPac, SportMix, CanineX…
Ever wonder how Nicci & our team at NorthPoint Pets (NPP) evaluates brands to determine what foods end up on our shelves? It’s actually a lot of work and involves a lot of tough conversations. We thought it would be fun to show you a little behind the scenes of what questions we ask and why, so you know how choosy we are when it comes to the food that ends up in your (and our) pets’ bowls! These questions we ask prospective brands ensures the quality and safety of our products.
Recently a pet food company reached out to us in an attempt to earn a spot on our shelves. In response, Nicci asked a series of questions regarding the quality and safety surrounding the food. The following is a dialogue between her and a Prospective Pet Food Company. (Please note that additional information is provided for context.)
Prospective Brand: We consult with a number of veterinarians and animal nutritionists who provide input on formulations as well as ongoing data from health and lab results for generations of dogs and cats on our food. We feel this is more objective than having a small team of nutritionists or veterinarians on our payroll and gives us access to a broader group of experts and studies. We formulate blends that meet (and often exceed) AAFCO Nutrient Profiles for dogs & cats and use only ingredients that have been proven through independent studies to have long-term health benefits and are the most digestible for the intended species.
NorthPoint Pets: This didn’t answer the question: who, and what are their qualifications? The veterinary nutritionist or Ph.D. nutritionists do not have to be employed by you, but their experience and qualifications are important to know considering there are so few of them in practice. Further, there is a big difference between a veterinarian and a veterinary nutritionist or board-certified nutritionist because veterinarians alone generally do not have the expertise or experience in formulating pet foods, so a veterinarian and/or Ph.D. with nutrition qualifications is important.
Prospective Pet Food Brand: (Did not further respond to this question)
Prospective Brand: All our ingredients are USDA/FDA certified to meet USDA nutrient profiles and FDA quality and potency standards. A Certificate of Analysis (COA) is provided for every ingredient which includes test results for a variety of standards including heavy metals.
NorthPoint Pets: The FDA doesn’t certify ingredients. Do you conduct any independent testing outside of the COA that you receive to ensure the ingredient levels are accurate? (Example, Hill’s wasn’t testing their vitamin D which led to their recall)
Prospective Pet Food Brand: (Did not respond)
Prospective Brand: Yes, we trace every ingredient and every supplement back to its origin.
NorthPoint Pets: Ok – but are you able to provide that information? And, do you source from China?
Prospective Pet Food Brand: (Did not respond)
Prospective Pet Food Brand: Our nutrient profiles are all substantiated and include all AAFCO essential nutrients, plus others that we feel are relevant, in both AS FED and DRY MATTER values. Our profiles are published on our website:
NorthPoint Pets: The information provided on your website is incomplete and unclear. For example, are these typical or targeted analyses? A typical analysis would provide the actual analyzed values of the formulas while a targeted analysis is just a prediction. Using predictions is not something that NorthPoint believes is safe or accurate. Additionally, how often are your formulas tested? These links don’t say.
Prospective Pet Food Brand: Did not respond.
Prospective Brand: We use ingredients that have published results for these tests, to the extent they are available for human consumable ingredients. As a company, we do not perform caged trials. We have 14 years of proven history with multiple generations of pets on our exclusive diets.
NorthPoint Pets: Using the individual values for each of these ingredients is not the same as a full digestibility trial for each formula because individual values do not count for processing losses, ingredient/nutrient interactions, or even how fiber impacts the bioavailability of various nutrients. Further, utilizing data available from human databases is not always accurate or translatable to pets as these also do not account for ingredient interactions and processing losses.
Caged trials are not necessary for digestibility values. For example, many companies feed the food to the employee’s pets and collect stools from them to determine digestibility values. That said, caged trials in my opinion are better than no digestibility values because without digestibility trials you are essentially experimenting on your client’s pets. It is also important to note that longevity in the marketplace does not prove adequacy. A great example of this would be Midwestern Pet Foods who has nearly 100 years and was recently the subject of multiple recalls due to significant ingredient sourcing and food safety gaps.
Prospective Pet Food Brand: Our facility is licensed for human food manufacturing and we are under continual USDA inspection at human food standards. In our 14 years, we have never had a recall or a single quality issue or failed an inspection/audit.
NorthPoint Pets: USDA inspection or human grade products/ingredients provide no guarantees. The most recent example is the Tyson chicken recall (8.5M lbs over 4 months) which has now impacted NomNom. Another example would be the Listeria recall for Just Food For Dogs which stemmed from ‘human grade’ green beans. These USDA designations are also not 3rd party verifications or food safety programs.

Prospective Pet Food Brand: Because we use real food the values for our ingredients are substantiated and published by USDA. We can provide actual USDA substantiated values upon request for all our food ingredients.
NorthPoint Pets: I defer back to my response to your answer to question 5, this is not an adequate response because without digestibility values you really don’t know where your calories are coming from or how they are assimilated. Additionally, USDA values provide caloric values based on Atwater – and modified Atwater is used for pet foods. It is also not a secret that Modified Atwater (or Atwater) has never been validated in dogs or cats which means this is a highly inaccurate way to estimate the calorie content or digestibility of your food or individual ingredients in your food.
Prospective Pet Food Brand: Did not respond.
Prospective Pet Food Brand: We adhere to all USDA HACCP and testing standards for human food manufacturing. This includes continual testing of all food surfaces throughout the manufacturing process as well as ingredients and finished products. Our meats are gently but fully cooked to minimum temperatures recommended by FDA for food safety, and we are required to keep detailed baking and testing logs for every tray. We follow similar logging and testing for raw ingredients. The disparity between feed standards and food standards is impossible to detail in a 2-page response, and we operate at or above the human food safety standards for manufacturing and finished goods.
NorthPoint Pets Comments: This is a fair response, but not great. This is because it does not indicate that their facility is indeed human-grade, shows they do NOT have a 3rd party safety program, or directly answer that they hold their products until testing to ensure that pathogens are not being released into the marketplace. Simply cooking products does not negate any risk of pathogen contamination as evidenced through pathogen contamination of cooked diets like kibble.
Overall, you can see that the responses by this company SEEM good, however, once we break down the terminology and provide some industry insight it is easy to see that this company is less than transparent. The problem is unfortunately not isolated to this pet food company. In fact, it is a pervasive issue in the pet food industry. Pet food companies have become increasingly savvy marketing companies providing carefully crafted answers and terminology that make pet parents and retailers feel good about food when in fact they are no different than some of the others that have caused issues in the past.
This example should serve as a good example of why it is so important to ask your pet food company really important questions and KNOW what the right answers are and what a deceiving set of answers looks like. Unfortunately, it does take a little bit of work, and the above answers can even fool even the best veterinarians. Nutrition and pet food really is a complicated industry – but we’re here to help you navigate it! If you have any questions please do not hesitate to stop in and engage our talented and passionate team.
….and other lessons courtesy of Midwestern Pet Foods
If you’re just catching up, Midwestern Pet Foods (manufacturer of Earthborn, ProPac, SportMix, CanineX, Venture, Unrefined and Wholesomes) rounded out 2020 with an expansive ‘voluntary’ recall’ secondary to aflatoxin contamination among a variety of their products. As 2021 began, the ‘voluntary’ recall became larger, as retailers and consumers we were assured that this incident was isolated to one of their four US plants. If you are wondering why I put ‘voluntary’ in quotes, it’s because voluntary recalls are actually not voluntary – they are forced by the FDA (For more on that, see my other article ‘Voluntary Recalls Are Not Voluntary’ here). In short, when a company is subject to a voluntary recall it appears to imply that either the company did not have to recall the product, or that they may have found the issue themselves and thus recalled the product ‘out of an abundance of caution,’ when in fact that is usually pretty far from the truth.
In the case of the aflatoxin recall with Midwestern Pet Foods, the recall was initiated by the Missouri Department of Agriculture as a result of pet illness reports and not Midwestern Pet Foods identifying the issue. Looking at the timeline of how things progressed, the initial recall was announced on December 30, 2020, expanded on January 11, 2021 (to include over 1,000 lots of pet food) and was followed up by an updated outbreak and advisory on January 26, 2021. The timeline and expansion of the number of lots and amount of pet food impacted shows just how important it is for pet owners and retailers to ask proper questions of their manufacturers to ensure the products they stock or feed their pets is safe – and to protect their businesses and livelihoods. In other words, the recall started out small and seemingly to be a self-caught issue, however the fairly quick expansion tells a different story. If Midwestern Pet Foods was properly testing inbound ingredients (i.e. aflatoxins in grains), testing foods after production and prior to leaving the facility (positive release) this would have never happened.
The outbreak and advisory notice outlines how the FDA was working with 10 different states to determine the impact of the aflatoxin contamination of Midwestern’s portfolio of brands. This advisory also provided an update that included at least 35 additional countries where the foods were likely distributed to and thus impacted by this recall in the United States. For example, The Food and Drugs Authority in Ghana issued a directive for all foods manufactured by Midwestern Pet Foods to be returned to the importer due to dangerously high levels of aflatoxin. For context, some of the products tested contained aflatoxin at levels as high as 558 ppb. FDA considers that aflatoxin levels in dog and cat food above 20 ppb will support a charge of adulteration because it is fatal or injurious to the health of pets.
Soon, the general public, retailers and veterinarians alike realized that this was not an isolated incident and in fact was a result of widespread food-safety issues and problems across all four plants owned and operated by Midwestern Pet Foods. The worldwide aflatoxin recall was almost immediately followed by a second recall due to Salmonella contamination of several other lots of products in March of 2021 made in a second Midwestern Pet Foods facility. If you read the recall notice on the FDA website, you’ll note that this recall is also quite expansive involving several recipes and brands. When recalls are this large, several questions should be raised which include but are not limited to:
Although the company claims that Salmonella-contaminated product never made it into the marketplace, and they caught the recall prior to it leaving their facility, the FDA letter states that it did indeed get distributed into interstate commerce. If none of the contaminated product left their facility and they were able to provide documentation to prove that no other product was impacted, then a recall would not have been triggered. This fact tells us again that food safety was an issue, and that again we see a large transparency problem with another manufacturer in the pet food industry.
The FDA Warning Letter issued to Midwestern Pet Foods on August 9, 2021, included five FDA Form 483’s. An FDA Form 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed any conditions that in their judgment may constitute violations of the Food Drug and Cosmetic (FD&C) Act and related Acts. I’ve heard a variety of comments after this warning letter was issued from pet owners and retailers – the largest, and most concerning is that many believe that because no new recall was issued that the Warning Letter is not really a cause for concern. Nothing is further from the truth and in fact, I would argue that the Warning Letter – impacting all 4 locations – is worse than a recall. Why do I say that?
This Warning Letter was issued as a Corporate Warning Letter because at the conclusion of inspections at all four facilities the FDA issued these FDA Form 483’s due to documented issues at each of their facilities:
“At the close of each inspection, you or your plant manager was issued a Form FDA 483, Inspectional Observations. We acknowledge you1 have provided written Form FDA 483 responses dated February 25, 2021 (OK), March 12, 2021 (NY), March 19, 2021 (IN), March 30, 2021 (IL), and May 6, 2021 (IL) describing corrective actions you have taken or plan to take to address the observations at each of your facilities.”
This shows that OK, IN and NY each received one, and IL received two FDA Form 483’s for a total of five. The problem is that most people have never read? past the initial FDA in Brief that was released on August 17th, 2021. This letter detailed numerous apparent violations of the Federal Food, Drug, and Cosmetic Act that were shared across the four sites. The FDA states that these violations are likely to have contributed to the illness or death of hundreds of dogs resulting from high levels of aflatoxin secondary to violations of Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for Food for Animals regulation.
Per the FDA/CVM: The last of the four inspections concluded on April 16, 2021, and FDA immediately began the process of examining and compiling evidence from the multiple inspections into a corporate-wide warning letter.
Because the firm had voluntarily recalled the pet foods that had the potential to be contaminated with Salmonella or aflatoxin, because investigators provided initial feedback to the firm about their inspectional observations (FDA Form 483s), and because the Agency did not have evidence that violative Midwestern-manufactured product was currently in the marketplace, the FDA did not initiate a product seizure or injunction case.
However, because the conditions observed during the inspections indicated significant problems, the Agency took the step of issuing a corporate-wide warning letter.
This Warning Letter is far more than the FDA saying ‘Hey, clean up your act’ – this is a warning to ‘do better next time’. Instead, a warning letter is typically issued after an inspection of a plant or manufacturing facility that finds quality control issues and/or after consumer-related complaints as a result of injury or death. In this case, the warning letter started with consumer complaints, which triggered inspections at all four sites which then found further issues. These issues are outlined in this letter in which the FDA requests corrective action by Midwestern Pet Foods:
“During the IL inspection, you recalled approximately 104 products of dry dog and cat diets made in your IL facility from October 26, 2020 – November 12, 2020, February 1, 2021 – February 12, 2021, and March 15, 2021 – March 19, 2021, because your routine monitoring yielded Salmonella-positive results for pet diets manufactured on common equipment during those periods.”
The explanation of such food safety issues within this letter is what, in my opinion, makes this worse than a recall letter. Recalls are supposed to catch unfortunate accidents and rare occurrences of pet food adulteration. The highlights of these violations go far beyond a mistake, oversight, or single incidence of human error because they expand across multiple products, multiple lots on multiple dates of manufacture simply because they were common mispractice across all four of their manufacturing facilities. Further, it’s important to point out that these recalls were indeed so large, likely because of two reasons: poor food safety AND documentation of manufacturing. Was it likely that all lots impacted were contaminated? No, instead a large amount of product was recalled in an attempt to reduce the potential for any to be in the marketplace. On the other hand, is it likely that all contaminated products were found or recalled? It is uncertain, and this is because nowhere within the FDA reports does it state that all retained samples were tested for all products in the marketplace. It is unknown if all contaminated products were caught since aflatoxins are never evenly dispersed in grains, and instead you have ‘hot spots’. The hot spots are the reason why a robust and thorough screening program is needed for incoming raw ingredients, during manufacture and prior to distribution into the marketplace. In other words, if these programs are implemented properly, things like aflatoxin and salmonella are preventable. As a result, the FDA likely made a press release so consumers, retailers and veterinarians can be hyper-vigilant in seeing early warning signs of aflatoxin poisoning in case all affected lots were not recalled.
Within this letter, the FDA also states that voluntarily recalling product does not prevent the recurrence of such hazards (aflatoxin and Salmonella) in their products. Hence the need for Midwestern Pet Foods to respond to this robust Warning Letter with their plans to mitigate these risks, upgrade their Food Safety procedures in the future, document proper training and implementation of the programs (i.e. aflatoxin analysis and environmental Salmonella detection). While Midwestern has responded to some of these violations, as outlined within the Warning Letter, there still remains more work to be done as well as additional inspections of all facilities to ensure compliance.
Not until the FDA inspects the facilities to ensure that their corrective measures are implemented and being executed properly, will the Warning Letter be resolved. In the meantime, since the warning letter and investigation are still open, one would be prudent to be cautious of any product coming from any facility with an open or unresolved issue.
At this point, you may be wondering what happened to Hill’s Warning Letter that was issued as a result of excessive levels of Vitamin D. This massive recall was also global and resulted from Hill’s not following their own food safety procedures. This Warning Letter is also currently unresolved, likely due to COVID restrictions and the FDA not being able to adequately execute a robust inspection. One could also argue that regulatory authorities don’t have the capacity to police everyone all of the time, or that they may be letting such a large manufacturer get by for reasons we can maybe discuss at another time. Regardless, the lesson is that unless a warning letter is resolved you should probably find another pet food for your pet, or stock a different brand in your retail or clinic location.
An unresolved Warning Letter has actually never happened in the pet food industry, although it has happened in veterinary product and livestock industries. This is because pet food companies have historically complied with any changes that the FDA suggested after issuance of the Warning Letter. So, what happens if a pet food, treat, or supplement company fails to make adequate changes or fails to comply altogether? It’s likely that the U.S. Department of Justice would have to bring on an injunction on the FDA’s behalf. For an example of this, we’d have to look to human industry examples to determine what would happen…
Current Good Manufacturing Practices (cGMP) are historically what trip up food and supplement manufacturers. Failure of a company to conduct their manufacturing under such practices after being issued a Warning Letter would result in the U.S. Department of Justice doing what? on behalf of the FDA. Just one example, the FDA shut down Sunset Natural Products for manufacturing and distributing adulterated dietary supplements following a ruling by a U.S. District Court which issued a consent decree against the company. Under a consent decree the company is not allowed to manufacture or sell their products until the FDA determines the business is in compliance with the Federal Food, Drug, and Cosmetic Act. While this has not happened often, it is possible for companies to resurrect themselves post past? an event such as these, but it does take quite a bit of reinvention.
Per the FDA/CVM: injunctions have also been regularly filed against dairy farms that have introduced food containing illegal drug residues into the U.S. food supply. FDA initiates civil actions like seizures and injunctions for different reasons than criminal actions. Civil actions are brought to protect the public from harmful products under FDA’s jurisdiction. Criminal actions are brought to punish culpable parties for wrongful action and discourage the behavior. Given the different goals and legal requirements for each, there are different procedures that FDA follows to initiate each type of case. Please see Chapter 6 of FDA’s Regulatory Procedures Manual for FDA’s policies about pursuing seizures, injunctions, and prosecution of criminal matters.
Depending on the nature of the violation, it is the FDA’s general practice to give individuals and firms an opportunity to take voluntary and prompt corrective action before it initiates a judicial action. In some cases, however, judicial action is necessary to protect public health. As noted above, all judicial action must be pursued by FDA through the U.S. Department of Justice.
Based on this and other incidents we can hypothesize that if a company did not adequately respond to or respond at all to a Warning Letter, the FDA would take legal means to shut down operations of that pet food company. In this case, involving Midwestern there are a number of issues across all of their facilities indicating that substantial overhaul of their cGMP is needed. If this does not happen, or happen to the level of satisfying the FDA, we could potentially see an injunction against the company – although this would be a first for the pet food industry.
At the end of the day, this is not just about Midwestern Pet Food, because there are multiple recent examples of adulterated pet foods in the marketplace that include Evanger’s, Sunshine Mills, Hill’s Pet Nutrition, Blue Ridge, NomNom…I could keep going. The lesson is just another example of why it is so important to ask pointed and meaningful questions of your pet food manufacturers. These questions include but are not limited to:
Bottom line: If your pet food company cannot answer these questions or they say the information is proprietary, don’t feed their food!
Nicole Cammack
Nicole founded NorthPoint Pets & Company to fill a void for pet parents: information and transparency. Nicci understood that, while there are countless pet stores and unending opportunities for buying online, much of the information about pet food and health is incomplete, biased, or misleading. Since 2014, she is proudly leading an incredibly talented team that boasts several national awards as the leader in independent pet retail, innovation, education, health, nutrition and transparency.
Research & evolution are the foundation for everything NorthPoint stands upon. Currently, Nicole is working on her PhD at the University of Georgia (UGA), College of Veterinary Medicine in Canine Nutrition & Metabolomics. In short, she is studying how the way we feed our pets can influence disease. Although research is not a new field for her, as she has experience in clinical research for diseases such as obesity, diabetes, Alzheimer’s Disease and various cancers. Her undergraduate and graduate education includes biology, chemistry, business and nutrition. She has worked in the pharmaceutical industry on multiple R&D projects and has had the privilege to learn from leading international figures in both the human and pet health industries. When not at NorthPoint or UGA she can be found presenting at national conferences, including federal, state, and municipal organizations. Her most recent publication reports on the prevalence of reported pathogenic infections related to raw pet food diets.
The Covid-19 pandemic has caused significant disruptions in the availability of pet food. If you rely on canned food for your pet, you may have noticed frequent out-of-stock situations at larger retailers. However, shopping at independent pet stores can offer a solution and support local businesses. In this article, we’ll explore the reasons behind supply chain disruptions, including labor and supply shortages, imported ingredient delays, and inclement weather. We’ll also discuss the increased demand for pet food due to higher pet adoption rates. Additionally, we’ll address the possibility of formula changes and provide guidance on finding assistance for out-of-stock pet food.
Large retailers such as supermarkets, big box stores, and even online retailers seem to have more significant out-of-stock problems than smaller independents. For you, this means that shopping at local independent stores could benefit you and the community you live in!
The Covid-19 pandemic has caused interruptions at every level of the supply chain, impacting various aspects that affect the availability of canned pet food. These disruptions, some of which may not have been previously considered, can take several weeks or months to manifest on store shelves. Let’s explore the key factors contributing to the slower production rates of canned pet food.
Labor shortages have had a significant impact on production, affecting livestock farms, canning facilities, and delivery drivers. These shortages have resulted from social distancing measures, lower facility capacities, and individuals needing to self-quarantine due to infection or exposure to the virus. With multiple instances of labor shortages, production lead times have increased from 1-2 weeks to 2-4 months. Consequently, some canned pet food manufacturers are currently unable to accept purchase orders. Certain brands or entire product lines may never resume production.
Supply shortages and delays are also contributing to the disruption. Livestock farms and slaughterhouses at the beginning of the supply chain have faced difficulties finding workers to process the necessary ingredients for pet food. Consequently, this has led to slower supply or temporary shortages. While there are alternative suppliers for common proteins like beef and chicken, more exotic options often experience long-term shortages.
Imported ingredients have encountered delays as well. Both national and international production facilities have implemented measures that require extended closures for quarantine, cleaning, and labor and supply shortages. Additionally, staffing shortages and safety measures enforced by the U.S. Customs and Border Patrol have prolonged the time imported products spend at ports, further exacerbating delays.
Inclement weather has also impeded transportation throughout the supply chain. Widespread delays caused by hazardous travel conditions and power outages resulting from winter storms have affected suppliers, production facilities, and retailers. Although this factor may not be the primary cause for empty shelves, it contributes to slower replenishment.
Many families took advantage of the extra time they’ve had the last couple of years and welcomed a new pet into their home.All those new pets inevitably brought a surge in demand for pet food. APPA reports 70% of U.S. households owned at least one pet in 2021, and pet owners have become increasingly interested in what they are feeding their pets. Wet and canned food, in particular, saw a dramatic increase in sales.
Some pet food manufacturers have chosen to discontinue or reformulate their recipes to bypass scarce ingredients to overcome some of the supply chain’s hurdles. So if your pet hesitates to eat his dinner, there may have been an ingredient swap that occurred. Be sure to check your pet food label to find possible ingredient substitutes.
If your pet food is currently unavailable, you likely have several questions in mind. Why is my pet food out of stock? Will it be restocked, and when? What alternatives are available for my pet’s dietary needs? Who can assist me in finding a comparable option?
Unfortunately, employees at Big Box stores are unlikely to provide answers because these large retailers do not have direct relationships with distributors. As a result, they possess limited or no information regarding out-of-stock items.
On the other hand, small independent pet retailers have the advantage of ordering and receiving products from multiple distributors. This proves beneficial when a particular supplier runs out of a specific item. Additionally, indie retailers maintain direct contact with distributors and enjoy close relationships with brand representatives. As a result, indie stores can provide accurate and real-time information sourced directly from the distributors themselves.
Moreover, indie pet store staff are well-equipped to assist you in finding suitable replacements if your preferred pet food is unavailable or discontinued. These recommendations are tailored to your pet’s needs, ensuring that they are not driven by sales goals but rather genuine concern for your pet’s well-being.
The below charts show a significant dollar amount increase for both dry and wet pet food formats. Dry food experienced a greater dollar growth; however it was actually a smaller percentage of growth in comparison to wet foods (data as of August, 2020). This means that there likely was more of a strain put on wet food producers, further adding to shortages.[vc_column width=”1/2″]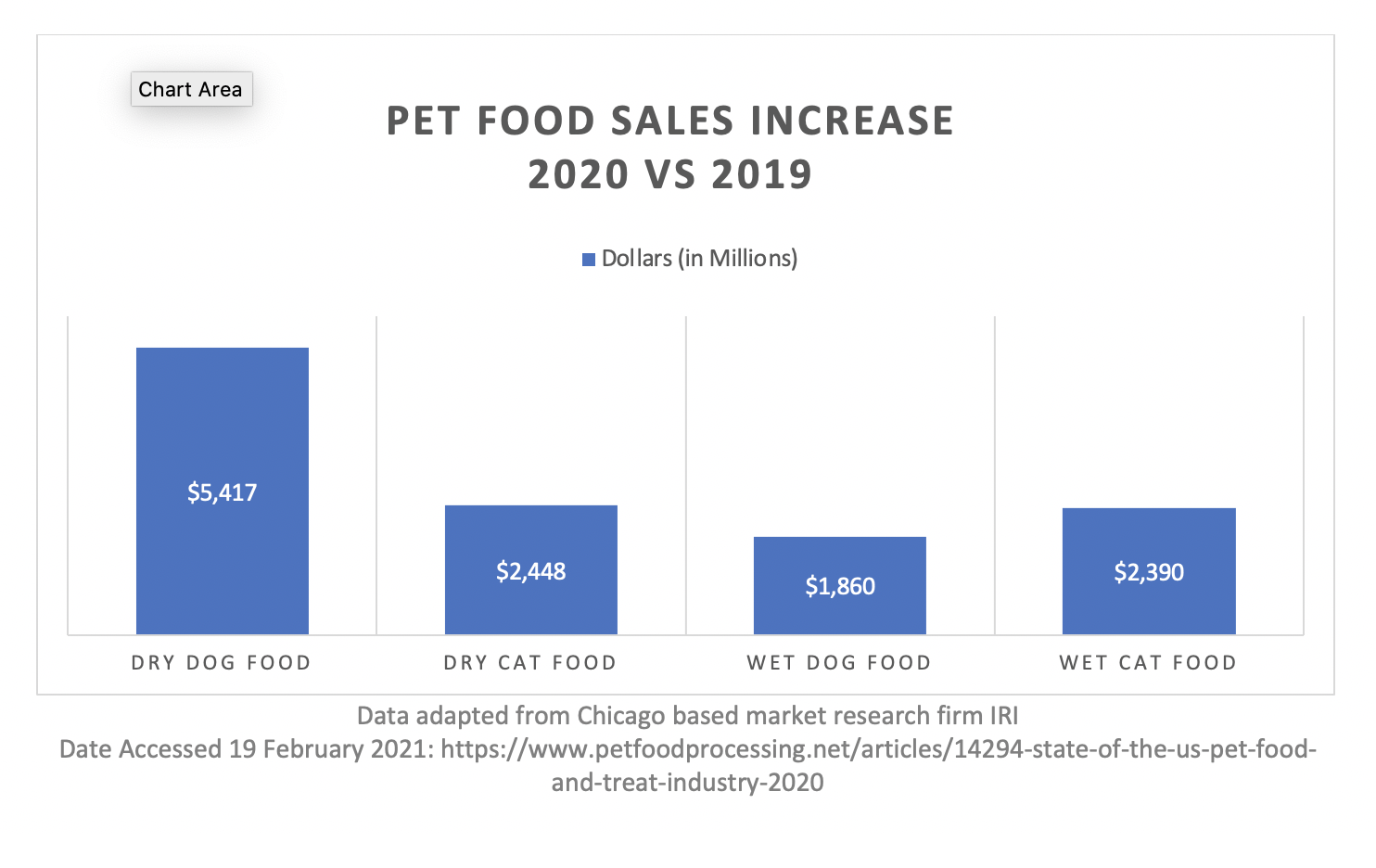 [vc_column width=”1/2″]
[vc_column width=”1/2″]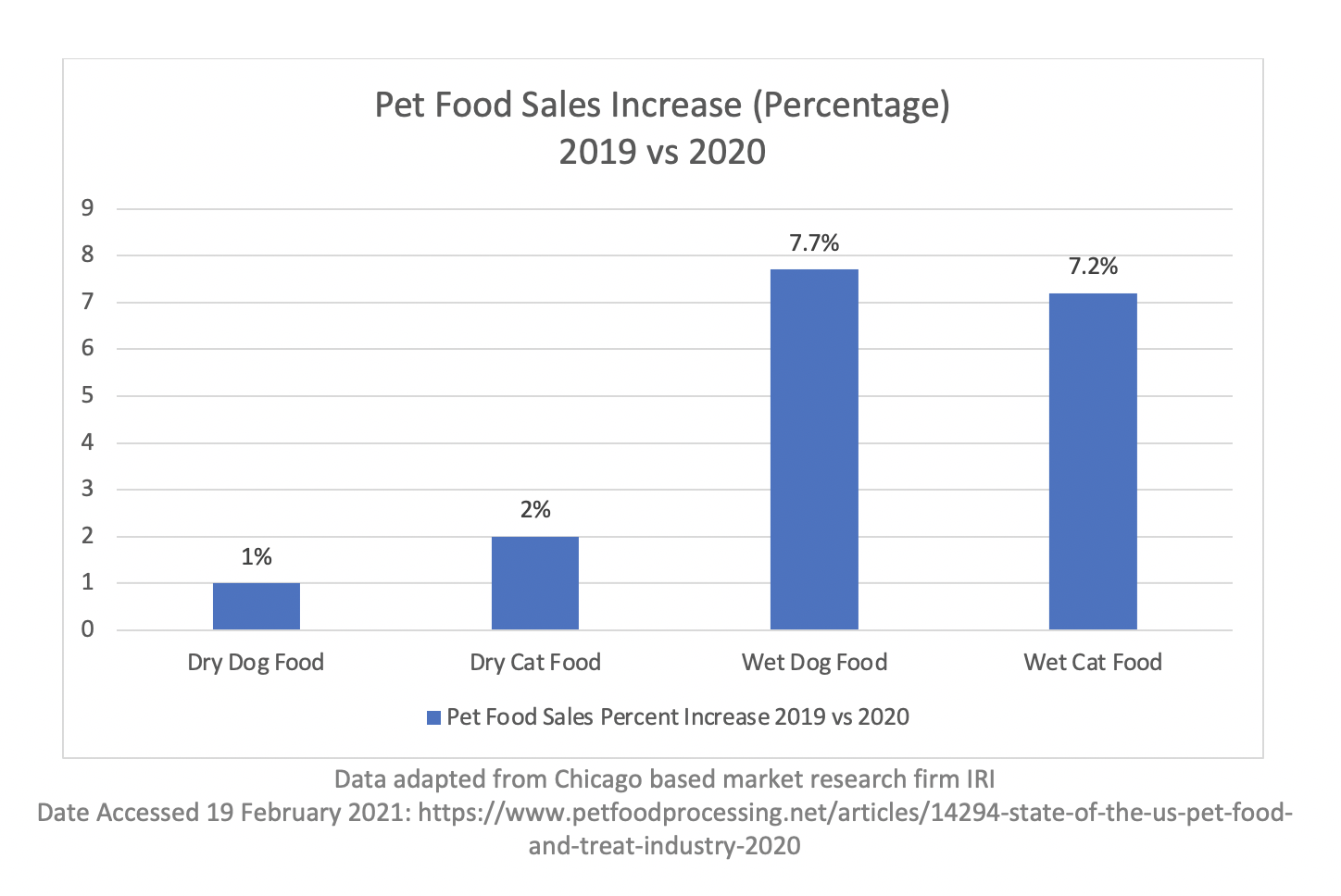
Nicole Cammack
Nicole is the founder & owner of multiple-award-winning NorthPoint Pets & Company, in Connecticut, USA. She has completed undergraduate work in biological sciences, business and holds an M.S. in Nutrition. Currently, Nicole is pursuing a PhD in Comparative Biomedical Sciences (Canine Nutrition/Metabolomics) at the prestigious University of Georgia in the USA.
Her background includes experience in the pharmaceutical industry on multiple R&D projects. Nicole has had the privilege to learn from leading figures in the human and pet health industries. Nicole has been heavily involved in police canine nutrition within the USA, helping to improve the modern care and feeding of working dogs. Her interests include working dog nutrition, raw feeding, pathogens, metabolomics, and nutrition’s relationship to disease in humans and canines. Her current research involves the exploration of the canine urinary metabolome and the relationship to diet.
Publications: Cammack, N.R., Yamka, R.M., and Adams, V.J. (2021). Low Number of Owner-Reported Suspected Transmission of Foodborne Pathogens From Raw Meat-Based Diets Fed to Dogs and/or Cats. Frontiers in Veterinary Science 8. doi: 10.3389/fvets.2021.741575.
https://www.frontiersin.org/articles/10.3389/fvets.2021.741575/full
Contact:
https://www.linkedin.com/in/nicole-cammack-8400084b/?trk=author_mini-profile_title
Jenna Harrison
Jenna enthusiastically joined our team in early 2021 bringing nearly a decade of pet industry knowledge and experience along with her. She is a proud mom to cats Aerie and Spook who are both credited with her interest in pet nutrition. Quickly Jenna realized that there was a lot to be desired for honest, unbiased and accurate information within the industry and she knew she wanted to help change that. Much like the team at NPP, she believes in the value individualized diet, fresh food and tailored advice can provide for overall health, regardless of age. She also is the mom to 4 sugar gliders, Crumb, Crosby, Bindi and Gatsby which helps bring additional small animal knowledge to our robust team. When she’s not helping pet parents at NPP Jenna can be found hiking with her husband Adam, horseback riding and painting pet portraits.
https://todaysveterinarybusiness.com/pets-appa-survey-covid/ https://www.avma.org/resources-tools/animal-health-and-welfare/covid-19/covid-19-impacts-food-production-medicine https://www.petfoodindustry.com/blogs/7-adventures-in-pet-food/post/9165-how-prevalent-are-covid-19-pet-food-ingredient-shortages https://www.americanpetproducts.org/pubs_covidpulsestudy.asp https://foodindustryexecutive.com/2020/12/pet-food-and-treat-sales-stay-strong-in-an-unpredictable-year/#:~:text=In%20fact%2C%20US%20pet%20food,up%20by%20more%20than%207%25. https://www.petfoodprocessing.net/articles/14294-state-of-the-us-pet-food-and-treat-industry-2020
Many pet owners, retailers and veterinarians believe that ‘voluntary’ pet food recalls show that pet food companies have caught problems as a result of quality and safety checks. However, this is mostly false. In fact, some of the largest and most dangerous pet food recalls were not caught by the pet food manufacturer – and instead a regulatory authority. So why are these recalls labeled as ‘voluntary’?
Meriam Webster’s definition of Voluntary is as follows:
Voluntary (adj.):
1: proceeding from the will or from one’s own choice or consent
2: unconstrained by interference
When it comes to pet food; however, many pet food recalls are not a result of the manufacturer finding a problem (e.g. mis-formulation, toxicant, etc.) like most people believe. In fact, some of the largest ‘voluntary’ recalls were not in fact voluntary even though that word is often used with FDA announcements and manufacturer press releases.
The truth is that most ‘voluntary’ pet food recalls are indeed forced, usually by a state department of agriculture and/or the FDA. In order to determine if a recall was truly voluntary you have to read the FDA announcement as well as put a few pieces of the puzzle together. The problem is that most consumers, retailers and even veterinary professionals are completely unaware that most of these recalls are not voluntary. What is worse is that many seem to believe that if a company issues a ‘voluntary’ recall it somehow absolves them of any wrongdoing – even if the problem was truly their fault. In short, voluntary recalls DO NOT mean that the manufacturer caught the problem – it usually means that some other regulatory agency (state department of agriculture, FDA and/or CDC) was made aware of an adulteration or illness and is forcing the manufacturers hand. Usually if a manufacturer fails or refuses to cooperate then involuntary recalls or FDA advisories and press releases are issued, but they are rare. An example of such an incident would be the Answers Pet food recall involving Salmonella detected in their straight beef formula. In this case the FDA issued an advisory because Answers (Lystn, LLC) recalled the affected lot in Nebraska, but did not implement a nationwide recall.
So how do you do know where to look to determine if a recall was really voluntary? And how do you know where to look?
Let’s look at some examples in recent pet food history:
April 6, 2012 was the initial announcement of a ‘voluntary’ Diamond Pet Food recall due to detection of Salmonella. This is because the pathogen was detected by the Michigan Department of Agriculture & Rural Development on April 2nd, 2012. While the FDA announcement and media outlets labeled this as ‘voluntary’ it surely was not. Ultimately a CDC investigation, in collaboration with other agencies, used DNA fingerprinting of a Salmonella strain infecting humans to ultimately trace it back to a specific Diamond Pet Food manufacturing facility. In other words, Diamond did not detect the Salmonella pathogen on their own simply because they were not adequately testing inbound ingredients and outbound products. Additionally, Diamond was not keeping adequate records regarding ingredient tracing, manufacturing schedules, cleaning schedules or retaining properly labeled samples of products manufactured. In other words, this could have been prevented if they followed proper procedure. As a result 49 human infections of Salmonella Infantis and over 50 pets sicked and at least 6 pet deaths.
This recall expanded to numerous expansions of brands made by Diamond including Diamond Naturals, Taste of the Wild, Kirkland, Solid Gold, Wellness, Natural Balance, Canidae just to name a few. However, foods manufactured but sold under different brand names were not recalled until after the CDC reported the outbreak of human illnesses which also were reported in Canada. Which should have been a lesson for other manufacturers to do their due diligence.
Evanger’s Pet Food ‘Voluntary’ Recall (Pentobarbitol):
February 3, 2017 was the initial ‘voluntary’ recall of what would be multiple Evanger’s canned pet food products due to adulteration with pentobarbital – a euthanasia drug. Initial reports of ill dogs surfaced at the end of 2016, although it took over a month for the ‘voluntary’ recall being issued. Expansion of the recall was announced on March 2, of 2017 that included all ‘chunk’ beef canned products made by the company. Another problem being that Evanger’s is a co-manufacturer – meaning they made canned pet food for other brands leading to this incident and previous incident possibly effecting other brands. This recall was also not ‘voluntary’ as Evanger’s did not detect the pentobarbital, and it’s also important to note they delayed recalling additional product. It doesn’t stop there though, because the ‘adulterated’ product actually turned out to be horse meat and not beef.
Taking a trip down memory lane, Evanger’s has had significant and widespread sanitary and food handling problems at their facility dating back to 2006. This was evidenced in citations and a lawsuit filed by the Village of Wheeling which resulted in restitution payments. Further, other significant problems were regularly in the news from 2008-2017 surrounding both Evanger’s products and illegal business activity. These issues included Fair Labor Standards Act violations, utility theft, theft and money laundering. On the product front further citations, FDA inspections, permit suspensions, warning letters for sanitary conditions, adulterated products, misbranded products and contaminant recalls. This long chain of issues was rounded out by the 2017 pentobarbital contamination incident.
January 31, 2019 Hill’s ‘voluntarily’ recalled numerous lots of canned pet food due to toxic levels of vitamin D. Ultimately two additional recall expansions were also released on March 20th, 2019 and again on May 20th, 2019. This recall turned into one of the largest pet food recalls worldwide with over 22 million cans of pet food involved, and likely a large number of pets that became sick or died. The incident involving Hill’s was also not ‘voluntary’ because they did not detect the problem themselves, and in fact the FDA found Hill’s failed to follow their own safety and quality control procedures even though Hill’s attempted to place the blame on the ingredient supplier. The FDA issued a warning letter to Hill’s with highlights as followed:
In short – even though Hill’s Pet Nutrition is touted to be ‘one of the best pet food companies’ by organizations such as the American Veterinary Medical Association and other vet groups because of their research and development into pet nutrition, publication of peer reviewed papers and for employing veterinary and PhD nutritionist – those ‘attributes’ can’t save them if they don’t follow their own food safety, quality and formulation procedures!
On September 2, 2020 Sunshine Mills, manufacturer of several brands of pet food issued the first wave of recalls due to dangerously high levels of aflatoxin. On October 8th, the second announcement expanded the
recall to 18 brands that Sunshine Mills produced. Again, this recall was not ‘voluntary’ and was initiated after the Louisiana Department of Agriculture found elevated levels of the contaminant during routine testing. The department then issued a stop sale order on the product in the state of Louisiana, which then triggered the ‘voluntary’ recall by Sunshine Mills. This again is another example of a company either not testing inbound ingredients and/or outbound product for safety and adequacy. This is yet another example of a large recall that also could have been prevented!
Most recently on December 30, 2020 Midwestern Pet Foods ‘Voluntarily’ recalled several SportMix dog and cat foods due to dangerously high levels of aflatoxin. Note that this date is not too long after the Sunshine Mills recall, which should have been a clue to pet food manufacturers to ensure they were adequately testing their products and ingredients for safety and adequacy. In addition, Neogen releases contaminant reports for agricultural crops each week and because of these reports the industry was well aware of aflatoxins present from corn crops in various regions of the country. Aflatoxin, along with other common agriculture contaminants were present due to various conditions tied to the growing season. Given the Sunshine Mills recall likely from the same 2020 corn crop, Midwestern Pet Foods should have been even more aggressive in testing inbound ingredients (as should other companies using corn).
It’s likely that Midwestern Pet Foods was not inbound testing their ingredients for aflatoxin and/or outbound testing their final products. The larger problem is that Midwestern Pet Foods has indicated in the past that they do perform these types of early 2 weeks later on January 11, the recall was expanded to include well over one-thousand (1,000) lots of pet food, and additional brands. To date over 70 dogs have been reported dead and over 80 ill – which is likely to expand. Again – another deadly event that was completely preventable!
At this point you’ve probably realized a common theme – all companies were either not following their own established food safety procedures, or any quality control checks at all. You also likely noticed that none of the above companies caught their own issue – they were all discovered by outside agencies. This means that companies had released product into the marketplace without adequate food safety checks which resulted in numerous pet deaths and illnesses, and some even resulting in human illnesses. In addition, all companies issued expanded recalls, which further solidifies the point that these companies did not have or were not following sufficient quality control measures to ensure safety of their products nor knew the impact of the adulterated products in the marketplace.
The major point is that these were ALL preventable. If they the companies were testing inbound ingredients for aflatoxin or vitamin D the ingredients would have never enter their manufacturing facility. If they had positive release programs for pathogens like Salmonella, the foods would have never left their facility.
What all of this means is that pet food companies that do not have such measures in place are essentially playing Russian roulette with your pet’s health and safety (and sometimes your own health). It’s our responsibility as pet owners, retailers and veterinarians to contact our pet food manufacturers and ensure that they have appropriate procedures in place and that they follow them. When they do not follow the procedures and issues arise, we need to know why and what they are doing to prevent it again. We are human, mistakes happen. When they cannot explain or chose to ignore the questions we ask, why should we feed or sell their foods? The days of pet food companies lacking transparency in their ingredient sourcing and quality control are over – or at least they should be if we demand it.
Nicci is the founder & owner of award winning NorthPoint Pets & Company, in Connecticut. She is also the Founder & CEO of Undogmatic Inc. Her undergraduate and graduate education includes biology, chemistry, business and nutrition. She has worked in the pharmaceutical industry on multiple R&D projects and has had the privilege to learn from leading international figures in the human and pet health industry. She regularly lectures at national conferences, including federal, state, and municipal K9 events. Her current research involves identifying pathogenic risk factors and transmission among raw fed pets through a comprehensive worldwide survey.
The recall of SportMix dog and cat foods may impact you even if you’re not feeding the food being recalled.
UPDATE: JANUARY 25, 2021
The FDA released an update which indicated the recall has expanded internationally. Further, based on the number of reported deaths and illnesses as of this date this is likely the largest documented aflatoxin recall within the pet industry.
UPDATE: JANUARY 11, 2021
The FDA released an update on the original recall announcement adding over 1,000 lots of pet food manufactured by Midwestern pet. Affected foods were made in their Oklahoma facility. More than seventy deaths have been reported with an additional 80+ pets ill.
This recall indicates there are some clear quality control issues within Midwestern Pet’s manufacturing operations. As foreshadowed in the original article below, it likely meant that they were not inbound testing their ingredients for safety and adequacy. Midwestern was likely not outbound testing their final product for safety and nutritional adequacy either. The recall expansion supports this and we can expect the number of reports of ill pets to increase, as well as further expansion of this recall.
ORIGINAL ARTICLE: JANUARY 1, 2021
Recently a recall of SportMix dog and cat foods due to ‘potentially fatal levels of aflatoxin’ was announced by the FDA & Midwestern Pet Foods. SportMix is manufactured by Midwestern Pet Foods who also makes well-known brands Earthborn, ProPac, Venture, Wholesomes, CanineX and most recently their ancient grain food Unrefined. The first FDA update indicated 28 dogs reported dead, and at least 8 more ill, with 70+ ill and 80+ dead as of the second announcement. It’s likely the FDA announcements will result in more reported cases.
If you are feeding SportMix, you can check the most recent FDA announcement for lot and date codes to see if your food has been recalled. If your pet is ill, be sure to contact your veterinarian right away. You can learn more about filing a Pet Food Complaint with the FDA here: Report A Complaint.
The recall was prompted when the Missouri Department of Agriculture tested multiple SportMix products which contained very high levels of aflatoxin. Currently, the Missouri Department of Agriculture and the FDA are investigating the incident to determine how and why the foods contained such high levels of aflatoxin.
What Is Aflatoxin?
Aflatoxin is produced by a mold Aspergillus flavus. Aflatoxin is dangerous at high levels, although low levels exist in common foods we and pets eat. These foods include nuts and grains (including ancient grains!) such as peanuts, and corn. In pet food, the most common culprit is corn, however numerous recalls have been announced over the years for a variety of human and pet products.
The FDA states that pets are more at risk of aflatoxin poisoning because they do not eat a varied diet like humans do. In other words, the cumulative effect of eating food with already high levels of aflatoxin makes the situation worse.
What symptoms should I look for?
The FDA States:
“Pets with aflatoxin poisoning may experience symptoms such as sluggishness, loss of appetite, vomiting, jaundice (yellowish tint to the eyes, gums or skin due to liver damage), and/or diarrhea. In some cases, this toxicity can cause long-term liver issues and/or death. Some pets suffer liver damage without showing any symptoms. Pet owners whose pets have been eating the recalled products should contact their veterinarians, especially if they are showing signs of illness.”
Why this recall should concern you:
Aflatoxin at dangerously high levels in pet food is preventable from a manufacturing standpoint. If a manufacturer is testing their inbound ingredients and outbound testing their final product, dangerously high levels of aflatoxin should never make it to the marketplace. The fact that it has been found in 9 different lots of pet food is concerning and raises several questions:
Was Midwestern inbound testing their raw ingredients to ensure that they were safe? In this case, it is likely that the ingredient containing the aflatoxin was corn.
Note: The past year there were agricultural reports indicating high levels of aflatoxin in some crops, meaning that if Midwestern was purchasing from these regions they should have been testing for aflatoxin, and other contaminants more frequently.
Was Midwestern outbound testing their final products to ensure that they were safe and nutritionally adequate?
What types of quality control does Midwestern have in place to prevent problems like this from occurring?
What steps does Midwestern take to clean machinery and storage containers in an effort to prevent cross contamination to other products made in the same facility?
What other products were made in this facility during and after the recalled product was made?
Does Midwestern hold a sample of each lot of food produced so that it may be tested if issues arise such as this?
Another Lesson?
The recall of SportMix dog and cat foods is another lesson to both retailers and pet owners that it is important to ask questions of the brand of food you feed your pets. You can learn more about those questions here. I am well aware of many who think that I’m being unreasonable when asking the questions I ask, or pushing for changes in regard to food safety and nutrition adequacy testing – but the reason why I do it is because things like this are PREVENTABLE. Sure, implementing nutritional adequacy testing is inconvienent if you’re a manufacturer – but it’s worse when pets get sick or die because you didn’t implement that testing. As a retailer it’s inconvenient to have to constantly reach out to pet food companies – but it’s worse when a pet experiences a problem because I didn’t do my homework. It’s clear that many companies do not check all the boxes, but I can do my best to support companies that are doing their best to improve. I can also educate my clients and customers on who does and does not have certain safety/nutritional adequacy measures in place. I can also tell them who refuses to answer questions!
Simply said, knowing what quality control measures a manufacturer has or does not have can make a world of difference. While we don’t know if this recall will be expanded to other lots, or even brands – it is not out of the realm of possibility. For example, if Midwestern truly did have one batch of a contaminated ingredient such as corn, and does have proper quality control measures in place (e.g. proper cleaning of machinery and storage containers to prevent cross contamination) then other products may not be affected. If they do not have adequate measures in place (or failed to follow them) it is possible other products will be affected.
In either case, the question still remains: how did the contaminated food end up in the marketplace to begin with? Was it because they were not inbound testing raw ingredients, or outbound testing the final product or both? We’ll have to wait and see.

Original Recalled Products: Date accessed: 11 January 2021 https://www.fda.gov/animal-veterinary/outbreaks-and-advisories/fda-alert-certain-lots-sportmix-pet-food-recalled-potentially-fatal-levels-aflatoxin

Second Recall (January 11, 2021) Date accessed: 11 January 2021 https://www.fda.gov/animal-veterinary/outbreaks-and-advisories/fda-alert-certain-lots-sportmix-pet-food-recalled-potentially-fatal-levels-aflatoxin

Date code example: Date accessed 11 January 2021 https://www.fda.gov/animal-veterinary/outbreaks-and-advisories/fda-alert-certain-lots-sportmix-pet-food-recalled-potentially-fatal-levels-aflatoxin
There is a dedicated team of veterinarians that advocate for the safe, responsible feeding of fresh, raw food for dogs and cats. These vets and other professionals are members of the Raw Feeding Veterinary Society (RFVS). Nicci is also a member of this organization as well and you can learn more about the RFVS here: https://rfvs.info/about-us/

DIY Dog Wash closes one hour prior to store close.
Subscribe to get weekly tips, seasonal advice, and be the first to know about events, new products, sales, and more.

