You walk the aisles of your local pet store, or maybe you shop online – and you notice that your pet’s food, treats and supplements are marked “Made in the USA.” You think that this means it is free from any foreign ingredients, and therefore safe – right? Wrong. In fact, various ingredients can be sourced from anywhere in the world and then assembled (made) in the USA. And, suddenly we see how deceptive that “Made in the USA” claim can be.
Where It All Began: 2007 Melamine Recall
China immediately comes to mind as a danger when we think about pet food and supply sourcing. This is because the 2007 melamine pet food recall was a rude awakening for pet owners in the USA. Prior to 2007, not many pet owners thought about where their pet food, treats, or ingredients contained within them came from. In fact, most recalls the decade prior were small in comparison and limited to excess vitamin D, methionine, and aflatoxin contamination.1 At the time, the melamine recall was the largest and most widespread recall of pet food in history – resulting in over 5,300 different products being removed from American shelves and at least four-thousand pet deaths.2 The biggest takeaway from this recall was that it included over 150 brands – those from grocery channel all the way to ‘premium’ brands. Showing that many companies sourced the same ingredients from the same places and utilized the same manufacturers despite what their marketing claims said. This is ultimately what prompted pet owners to start asking questions.
What is Melamine?
Melamine is an industrial chemical with uses ranging from a binding agent, flame retardant and as a polymer for utensils and plates. It has also been used as a fertilizer, although it is also not approved for use as such in the USA.3 For context, melamine-related compounds in the same family include cyanuric acid, ammeline, and ammelide. Melamine and related compounds have no approved use as an ingredient in animal or human food in the United States.3 Just knowing these facts, the thought that melamine was even introduced into the pet food supply is unsettling and it was only by sheer chance that these products did not make it into the human food supply.
Recall Summary
The short version of the 2007 recall is that wheat gluten and rice protein were intentionally combined with melamine for its high nitrogen content. Higher amounts of nitrogen can cause the protein content of an ingredient to test higher than it actually is. In addition, cyanuric acid was also present and this combination of melamine and cyanuric acid is likely the reason the recall was so deadly – not the melamine on its own.3 This entire incident fueled the American pet owners obsession for ‘Made in the USA’ products. But are products that claim to be made in the USA void of ingredients from China? Maybe not.
Made in the USA – Sort Of
The melamine recall gave rise to the “Made in the USA” mark on pet food packaging, and the current Covid-19 Pandemic is fueling another wave of consumer demand for these products. But what many consumers and pet owners don’t realize is that this term does not mean sourced in the USA. Instead, it means an American company could make pet food on American soil, but source ingredients like vitamin and mineral mixes from outside the USA and still put “Made in the USA” or “Manufactured in X US state” on the label. Further, many manufacturers will claim – at least verbally, that they do not source any ingredients or vitamins from China. So, is this actually the case? No, because most pet foods on the market contain a vitamin premix, at least in part that comes from outside the USA, which many include China. More on that in a bit.
The Federal Trade Commission (FTC) does oversee ‘Made in the USA’ claims – either expressed or perceived. This claim according to FTC standards means that all, or virtually all of the product must be made in the USA and contain no or negligible foreign content.4 According to the FTC standards, sourcing for foreign vitamin and mineral mix would be considered ‘negligible’ and therefore allow a pet food company to use ‘Made in the USA’ on the label so long as a significant majority of the remaining ingredients were indeed US sourced. One grey area here is that ‘negligible’ is not explicitly defined.
Further, negligible doesn’t necessarily mean a small amount of the final product because the FTC also takes into account the economic factors of where ingredients are processed and where the final product is processed.4 Simply, a fair amount of ingredients could be sourced outside the US, but the economic impact of processing the final product could potentially outweigh the outside sourcing.
Misleading Consumers
Evidence of how misleading some companies could be can be seen in the recent case Weaver v. Champion Petfoods USA Inc., et al. The plaintiff in this case alleged that Champion Petfoods deceptively marketed their dog food products via claims such as “biologically appropriate”, “fresh”, “regional”, and “never outsourced”. In addition, he claimed that Champion should have to disclose trace amounts of BPA and pentobarbital – just for example.5 In short, the court dismissed all complaints because Champion Petfoods asserts that “biologically appropriate” is a nutritional philosophy – not a nutritional statement (aka a marketing tagline). Champion also asserts that they did not intentionally add BPA or pentobarbital to their foods – and also never warranted their products were free of these contaminants which releases them from any liability.
While rulings on all complaints favored Champion – it does not absolve them of the significant issues that have been exposed. It also doesn’t negate the fact that this is a loss for consumers who expect transparency and truth in what they are buying for their pets. The outcome of this case just proves the point that many of us in the industry often make: most pet food companies are marketing companies first, pet food companies second.
Ultimately this decision reinforces that manufacturers are not responsible for listing each and every material that may make its way into a final pet food product, nor are they required to be fully transparent on where and how ingredients are sourced.5 It also shows that many companies are not required, nor do they satisfactorily test inbound ingredients or outbound product for adequacy. Instead it provides examples of many of the marketing loopholes that manufacturers use to give the perception that the ingredients are superior, or sourced in one way over another – when in fact they are no different than other manufacturers.
Manufactured in the USA Loopholes:
One loophole that does not appear to have any regulation governing claims or advertising involves a phrase such as “Manufactured in our US facility” and/or “Manufactured in our Kentucky Kitchens”. Simply naming a US state is a gray area since this statement would be true, but it does not point to the sourcing of the ingredients. This is extremely deceptive to the consumer who is unaware of these details. Regardless, companies such as Tyson, Fromm, Wysong, Merrick, Wellpet (Wellness, Eagle Pack, Holistic Select) have had lawsuits dismissed as a result of this gray area – or due to gray areas under the FTC standards.6
There are multiple vitamin premix companies in the US that also source individual components from outside the US, including China. The way they get around that fact is they mill or blend the vitamin premix in US facilities and simply act as if as if ‘manufactured’ is the same as ‘sourced’ in the USA. Regardless, sourcing of a particular ingredient from the US or otherwise does not guarantee safety, and therefore doesn’t discount the need for adequacy or contaminant testing.
Understanding Origin:
If pet food or vitamin premix companies advertise ‘made in the USA’ they should be willing to provide a certificate of origin for each of the ingredients on their label. Interestingly, most companies we surveyed were not willing to provide this information. Opening the discussion about vitamin premix sourcing opens up another complicated layer of pet food industry safety, sourcing and transparency. Although, as discussed, a certificate of origin for a final pet food or premix product would not ensure individual ingredients did not originate from outside the country of origin. For example, if a vitamin premix is formulated and combined in Germany – it can still have individual vitamins that are sourced from China or otherwise. The certificate of origin for the final product would say Germany. This is because the country of origin is defined as the country where the last substantial economically justified working or processing is carried out. This is the very reason why pet food companies may not understand their product contains ingredients from China – although ignorance should not be an excuse.
Do Any Vitamin Mixes Come From the USA?
As of the date of this article, there is only one vitamin mix company claiming to be 3rd party verified to not source any ingredients from China.7 This sounds good, however, it is simply marketing because there is not an authority providing benchmarks for determining what 3rd party verification in this context means. In fact, there are several companies that perform the verification by sourcing and auditing. There are several non-China sourced vitamin premixes for pet food available in the market, but there is only one that advertises it. However – one must realize that there are some ingredients to these premixes that only come from outside the USA. For example, sources for L-carnitine only come from China or the Czech Republic, meaning that if a premix contains L-carnitine, that premix is not 100% USA sourced – despite any marketing claims. The same goes for some other vitamins and minerals as well.
Vitamin Premix Concerns
The above considered, there are reputable premix companies that have been sourcing ingredients from places other than China both before and after the melamine recall. Some even make the ingredients themselves ensuring control over the process. These companies are the ones that continually receive awards and recognition for their blending, sourcing and commitment to quality each year. These companies also have marketplace longevity showing an extensive safety record. Simply, they don’t have to advertise their USA sourced, or non-China sourced ingredients because their reputation and performance speaks for itself. Vitamin premix companies that hang on advertising claims without verification, performance, or data to back their claims are likely a disaster waiting to happen. This is something that pet owners and pet industry professionals should be cognizant of.
We’ve learned that most US pet food companies who claim not to use ingredients from China aren’t exactly transparent. For instance, when companies are specifically questioned about whether they source ingredients from China, they typically respond in one of two ways: either stating that their ingredients are sourced “globally” (which, by the way, includes China), or claiming that such information is proprietary.
The proprietary response is a problem because companies will use this to hide certificates of origin for various ingredients, or the premix itself (China or elsewhere), or the fact that they do not perform adequacy testing. What the big miss here is that not all ingredients from China are bad. Yes, I said it – not everything that comes from China is dangerous – so long as a company is verifying safety and adequacy. In fact, ‘USA’ made or sourced should never be an excuse to not adequately test products.
So Why Does ‘Everything’ Come from China?
The reality is that China actually owns and controls a vast majority of the vitamin and supplement market. The same goes for human vitamin products as well. Chinese companies were able to see the consumer trends toward health and wellness decades ago and purchased a large amount of businesses in this sector. As with anything else, there are bad players and there are some that go above and beyond – even exceed US quality in some instances. It’s common knowledge that some industrially produced vitamins and supplements from China pose significant risk. In addition, we know that this area is also a significant culprit for counterfeit medications. The concerns from this area are legitimate and created significant issues for countless people across the globe. However, these do not negate the fact that some legitimate supplies largely come from China or may be superior to others.
While high-quality ingredients coming from China may actually sound counterintuitive, we often forget that Traditional Chinese Medicine (TCM) dates back at least 23 centuries – likely much further. One of the foundational concepts of TCM is the balance of energy and includes the use of herbs, whole foods and other modalities to promote a healing effect within the body. While my description is oversimplified, the point is that those within the Chinese culture who embrace the philosophy of TCM believe in and understand the importance of high quality, clean and well-sourced raw materials and supplements. In other words, obtaining vitamins, minerals and supplements from reputable sources within China, or elsewhere, and then verifying quality and purity with 3rd party testing for use in pet food and human supplements can be safe.
The Problem Isn’t China
In fact, the problem is that we are focusing on the wrong target. This argument is often used to disguise significant issues of our own. Instead of looking to place blame on one particular thing, we should be seeking to hold ourselves and others accountable. Instead of simply blaming China, we should realize that the problem is actually twofold:
- Lack of raw ingredient testing/verification by American pet food companies
- The lack of legitimate transparency from American pet food companies regarding this testing
Poorly sourced and contaminated ingredients can come from anywhere. We have allowed those within other countries and the US to get away with providing subpar and dangerous raw materials for a very long time. These adulterated ingredients could be by error, accident, be innocent, or intentional. As discussed earlier, incidents have originated from the USA as well as China. Instead, we should be asking pet food companies how they ensure the safety of their ingredients and product as a whole. Those questions include:
Do you inbound test all ingredients for pathogens, toxicants and ensure the nutrient value is correct?
This question is important because it identifies whether or not a company has processes and procedures in place that would have prevented issues like the melamine incident and the recent Hill’s vitamin D recall. The pet food company or more likely the manufacturer that makes their products should have a Global Food Safety Initiative recognized 3rd party food safety certification (i.e. SQF, BRC, etc.). These certifications verify they follow procedures they have in place ensuring adequacy.
Do you conduct a 3rd party nutrition analysis on all of your finished products?
If the answer is yes, follow up questions should be: do those products meet an AAFCO nutrient profile? Are you willing to provide a copy of that analysis?
Note that most companies are deceptive and provide ‘target analysis’, which is what is predicted – not necessarily representative of what is in the finished product. Be careful with terminology here. In addition, beware of the word proprietary – this does not hold a strong argument and may be a tactic to hide lack of testing or inadequate testing. Most companies we surveyed either answered “no” or that this information was “proprietary”.
Do you conduct 3rd party digestibility studies for each of your formulas?
If the answer is yes, for any number of their formulations you should also ask if they make those publicly available. Again, proprietary is often used when this question is asked and that should be a red flag for any pet owner or pet store owner.
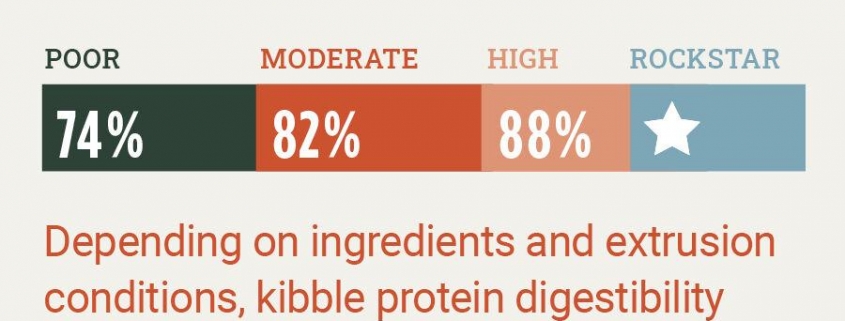
To assess digestibility, researchers feed a predetermined amount of the formula under study to several dogs for 14 days. Subsequently, they collect and analyze the stool to determine the percentage of absorbed macronutrients and micronutrients in comparison to the amount that was fed. This process does not involve any invasive procedures, inhumane conditions, or otherwise harm to the animal. Again, most of the companies we asked either refused to answer, claimed the information was proprietary, or indicated they do not perform this test. In short, companies that do not conduct digestibility trials remain unaware of the actual calorie content or digestibility of their food, which ultimately makes your pet the subject of experimentation.
Keep in mind that digestibility and typical nutrient analysis mean nothing without the other. Digestibility shows the amount of nutrients absorbed. The analysis shows the amount of nutrients contained within a product.
Do you source any ingredients from China? Are you willing to provide certificates of origin?
As we discussed, ingredients from China are not necessarily bad as long as they are well-sourced. Also, we now know that companies can and should be able to verify quality and purity via analysis and contamination testing of ingredients coming into their facility.
Do you complete an analysis of your final product to ensure the formulation is correct and ensure there are not any contamination issues?
Again, if companies were analyzing their final product both for nutritional adequacy and contaminants many of the memorable recalls and pet food scandals could have been prevented. The same examples apply – the melamine recall, the Hill’s Vitamin D recall and it’s also highly likely the dilated cardiomyopathy (DCM) and grain-free debacle would never have amounted to the mess it has with these quality control measures in place. In other words, if companies were able to 3rd party verify that their foods contained required nutrients at appropriate levels and that those nutrients were digestible then grain-free foods, taurine deficiency, or other factors would not have been ‘blamed’ for DCM.
Summary
Ultimately, dangerous ingredients and products can come from anywhere. Believing that because something comes from the USA provides assurance of safety is arguably irresponsible. It’s also arguably reckless for companies to advertise USA made/sourced products in an effort to provide the perception of transparency. Ff companies don’t have solid quality control in place – it’s not a matter of if, but when, and how big or deadly that recall will be.
Now, with our understanding that it is highly probable that a portion of your pet food originates from China, and that ingredients from other regions could also pose a risk, we realize that these risks are persistent and cannot be disregarded. Instead, it’s up to us to ensure we’re supporting companies who are doing the right thing.
References:
- Rumbeiha W, Morrison J. A review of class I and class II pet food recalls involving chemical contaminants from 1996 to 2008. J Med Toxicol Off J Am Coll Med Toxicol. 2011;7(1):60-66. doi:10.1007/s13181-010-0123-5
- Pet Food Recalls Spring 2007 – VIN. Accessed July 16, 2020. https://www.vin.com/AppUtil/project/defaultadv1.aspx?id=5715799&template=ContentOnly
- Medicine C for V. Melamine Pet Food Recall – Frequently Asked Questions. FDA. Published online November 3, 2018. Accessed July 13, 2020. https://www.fda.gov/animal-veterinary/recalls-withdrawals/melamine-pet-food-recall-frequently-asked-questions
- Complying with the Made In USA Standard. :42.
- Judge Dismisses Deceptive Labeling Claim Suit. Accessed July 18, 2020. https://www.natlawreview.com/article/deceptive-labeling-claims-based-trace-amounts-sent-to-dog-house?fbclid=IwAR3Qk40CojVyUFqFFozxkdcG0lsVlRBiGgd_3ompZ_5EUORUKMGGB3WkMIQ
- More phony “Made in the USA” pet food claims under attack; Multiple class action lawsuits filed | Poisoned Pets | Pet Food Safety News. Accessed July 17, 2020. https://www.poisonedpets.com/lawsuits-accuse-pet-food-makers-over-phony-made-in-the-usa-claims/
- Non-China sourced vitamin premix ‘first for North American pet food industry.’ Accessed July 14, 2020. https://www.petfoodprocessing.net/articles/13636-non-china-sourced-vitamin-premix-first-for-north-american-pet-food-industry








 [vc_column width=”1/2″]
[vc_column width=”1/2″]